|
In this Issue
Some Other Links
|
Editorial Dear ACGT newsletter readers. Happy New Year! Best wishes for a very nice and fruitful year 2010 The New Year is always a time for reflecting - a convenient benchmark for measuring what has been learned so far. In this edition, we are presenting an update on the status of the activities and the tools that have been developed by the ACGT consortium. In this respect, we are glad to announce the ACGT competition that will take place during 2010. The competition, described in this winter 09 newsletter, will be opened to all interested organizations and individuals to allow the use of the tools developed by the ACGT consortium. We are looking forward to collaborating with you on this major event. We wish you will have a pleasant reading through the articles and wish to encourage you to contact us for further collaboration and interaction with the ACGT project. Samuel Keuchkerian Clinical Trials Antigen scenario of the SIOP clinical trial (Norbert Graf - USAAR)
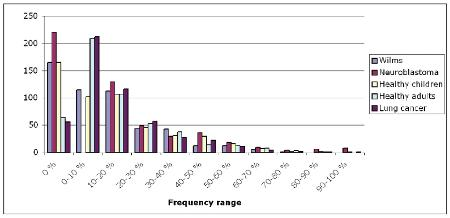
Wilms tumour is the most common malignant renal tumour in children. In the SIOP 2001/ GPOH trial clinical data, molecular data and pre- and post-chemotherapy DICOM imaging studies are collected, coming from patients out of more than 50 hospitals in Germany. Since 2009 anonymized data of the SIOP/GPOH trial are used in the ACGT scenarios. From a limited set of these patients, microarray data and data of autoantibodies against tumour specific antigens of Wilms tumour are provided. The main question is to answer whether molecular biology helps to define new risk groups in Wilms tumour and can be used to stratify treatment of these patients in the future. As ACGT promotes the integration of heterogeneous data and provides necessary analytic tools, it facilitates further molecular analysis and allows clinicians to efficiently analyze data that are presently communicated by mail, fax or maintained in flat text files at various remote clinical sites. One of the scenarios that are analyzed in ACGT is the Antigen scenario, to analyze if autoantibodies against tumour specific antigens do correlate with histology and outcome. Up to now in 133 patients we did receive serum for the Antigen scenario. Altogether 355 sera are collected from 265 patients out of 36 local hospitals. Out of this cohort 72 sera were from healthy children and 60 from patients suffering from other cancers than nephroblastoma. These sera are used as a control groups. Most of the sera are collected at the time of diagnosis. A preliminary analysis of the Antigen Scenario regarding the characterization of found autoantigens against nephroblastoma was reported at the Nephroblastoma meeting in Chamonix, France in March 2008 and at the SIOP conference in Berlin in October 2008. In contrast to adult patients one can find more autoantigens in sera of children. This is shown in figure 1. The same autoantigen can be found in a higher frequency in children and children with cancer than in adult cancer patients. 61 clones could be found that discriminates between nephroblastoma and neuroblastoma. 39 of these clones can be selected as best discrimators. Figure 2 shows for a single antigen the discrimination between sera of patients with nephroblastoma and neuroblastoma. [...] Products and Services Literature Based Discovery (Andreas Persidis - Biovista)
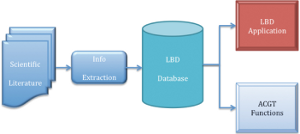
Literature-based discovery (or "LBD" for short) is a relatively new approach to knowledge discovery. It makes the assumption that by connecting seemingly disparate chunks of knowledge from within a relatively large corpus of scientific articles or other textual resources, it is possible to create new knowledge that does not exist in the original corpus. LBD is similar to data-mining, the difference being that while the latter deals with large bodies of numeric data, the former uses running text as it primary source. LBD has many applications, including the identification of biomarkers, predicting Adverse Events (AE) and finding new uses for existing drugs or compounds. Within ACGT, partner Biovista has developed versions of its proprietary LBD platform that are compatible with the basic ACGT infrastructure and can either be integrated in any workflow created by the ACGT Workflow Editor or incorporated in any other user application. The figure 1 above sows the basic architecture of the system. Information extraction algorithms read scientific articles that are downloaded from Medline on a regular basis ensuring the system is always up to date. Extracted information consists of about 25 classes of biologically relevant concepts, such as genes and pathways. Once extracted, these concepts are cross-correlated amongst themselves and these relationships stored in a custom-design database that ensures very high response rates. This database is then queried either by the LBD application or the LBD functions that are accessible via the ACGT infrastructure. LBD accuracyAs with any predictive system, one of the main concerns is its predictive accuracy; in other words how confident we can be in the output of such a system. To address this question a study was carried out and will be reported in reference 1. The study looked at Biovista’s LBD platform for predicting AEs before clinical trials, using abstracts from PubMed as the primary raw data source. Using a description of the mode of action (MoA) of a drug as the starting point, we compared it to the MoA underlying all AEs, for similarities. The dataset was 66 unique drugs, of which 61 were oncology, 7 were neurology, and three were both, where the AEs were reported at the American Society of Clinical Oncology (ASCO) annual meeting in 2007 and the American Academy of Neurology (AAN) annual meeting in 2008, respectively. The primary focus was oncology, where our sample covered 87% of the MoAs of all FDA-approved cancer drugs. Using data from 1997 to 2007 divided into five time points, and a total of 881 measurements, a mean of 79±22% of AE prediction was achieved. A similar AE prediction rate of 79±28% was achieved in the small neurology sample, in an additional 97 measurements (978 in total). We also found that when using data that pre-date any publication on a drug by five years, literature-based analytics predicted 72% of its AEs. The figure 2 shows how the predictive accuracy of the platform varies as a function of time (ie available data). [...] Grid news Toth - Distributed Logging for ACGT environment (Juliusz Pukacki – PSNC)
One of the most important paradigms for designing ACGT architecture is the idea of loosely coupled services cooperating with each other to provide desired functionality for the end user. That architectural model is the consequence of Service Oriented Architecture (SOA) approach chosen for ACGT. There are many advantages of using SOA solution: flexibility, lower maintenance costs, well defined integration schema, but there are also some drawbacks. One of them is the problem with monitoring and debugging of users actions in the distributed environment. The reason for that is quite obvious: single action on the level of user interface can cause multiple services invocations in the background. The best example of it, is the Workflow Environment where the user can design his/her experiment as a set of operations involving usage of databases or grid nodes. The crucial issue for the ACGT services developers is the ability to track the flow of actions initiated by the user throughout the whole system. To fulfill that requirement, Toth the distributed logging system designed by PSNC, was deployed in the ACGT environment. The main idea behind to Toth is to provide simple tools for the services to store the logs in the remote logs repository, and to provide simple yet powerful mechanisms for analyzing and filtering stored entries. The main assumptions taken into account during Toth design and implementation are:
[...] Feature article The data-sharing platform of the NeoBIG research program
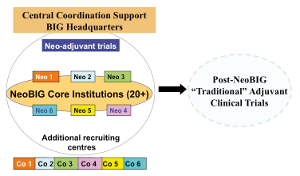
The NeoBIG program is a research program led by Breast International Group (BIG), and aims to organize and set up next generation clinical trials in the area of breast cancer R&D. A durable, multidimensional translational research structure supporting neo-adjuvant trials is expected to be built in order to share strategies, expertise, technologies, methodologies and protocols. In addition this will provide a strong foundation for future adjuvant trials in breast cancer (and research in other cancers). Tools and expertise developed in ACGT could be used to support NeoBIG, especially concerning the data storage, management and sharing, and with respect to privacy and security. In order to determine a possible support of the ACGT infrastructure, users requirements have been collected, scenario refined and finally the suitability of the ACGT tools and infrastructure to support the NeoBIG programme have been considered. The NeoBIG program will include several (five currently planned) neo-adjuvant trials that will be carried out together with various Pharma companies (see figure 1). A first trial is scheduled for 2010. Each trial involves several steps from the biopsy to the surgery, leading to various types of data that should be gathered electronically. To provide a platform that enables data sharing and collaboration between cancer research centres, NeoBIG requires a robust, secure IT solution that is compliant with a wide set of regulations and laws in the context of security, safety and privacy protection. The platform needs to be able to store, manage, and share the various types of data that will be generated by NeoBIG trials. [...] Community views Valencian Cyberinfrastructure for Oncological Medical Imaging (Ignacio Blanquer - Universidad Politécnica de Valencia)
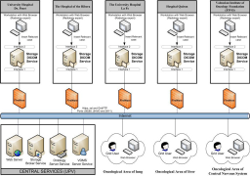
The generalisation of digital imaging has lead to the availability of a vast amount of knowledge in the form of medical images and reports, which is of enormous relevance to research and training. In these studies, data are retrieved and structured for healthcare delivery, around the identity of the patient. However, research and training requires organizing studies by content, setting up relations among images of similar or related pathologies, or morphological similarities. This cannot be achieved on current image databases, and moreover, the differences among centres will make studies incomparable. In this scenario, the project CVIMO (http://www.grycap.upv.es/cvimo) "Valencian Cyberinfrastructure for Oncological Medical Imaging (CVIMO GVEMP06/004)", funded by the Regional Ministry of Industry, University and Science of the Valencian Government, a middleware was developed and tested for sharing images and radiology studies from five hospitals of the Land of Valencia (Quiron Clínic, University Hospital Dr. Peset, de la Ribera Hospital, Valencian Foundation for Oncology and the Research Foundation of la Fe Hospital) with the collaboration of British Telecom. The project was leaded by the "Universidad Politécnica de Valencia" and the scientific coordinator was Vicente Hernández. [...] Events ACGT competitionThe ACGT Competition has been set up by the ACGT Project Consortium to encourage the creation of Grid enabled services that can be used for the support of multi-centric clinical trials and research. The ACGT Competition is open to all parties (academic groups, individual researchers, companies etc) that are interested in developing ACGT-compatible services and will take place between February and April 2010.
A WIKI is available for the competition entrants: http://www.biovista.com/ACGTCompetition/Main_Page. [...] Legal & Ethical Analysis of the Grid infrastructure and its implications on intellectual property issues (Marcelo Corrales - LUH)In the realm of a clinical trial scenario a Grid computing infrastructure has been identified as a key to support and facilitate the cooperation of scientists and resources through scalable computation and the management of data systems. The ACGT platform consists of multiple interconnected IT resources networks allowing users to execute a variety of scientific applications requiring a trustworthy, steady and prevalent access to computational capabilities. This complex collection of servers and communication protocols poses legal intellectual property questions: should copyrights or patents protect the grid? What about software licenses in a Grid environment? For a better legal analysis, it is important to know what the grid is. A Grid infrastructure is generally described with three different layers. The lowest layer is usually called "platform", consisting of the hardware resources such as computers, networks and interface devices which are geographically distributed, presenting their data in a variety of formats. The second layer, also called the “middleware“, is defined as the software layer that lies between the operating system and the applications on each site of the system. The last layer provides the user with application services including workflow engines, data visualization tools, semantic web and web portals. Intellectual property rights can be applicable to different aspects of the Grid infrastructure: [...] Life in ACGT Workshop on European-Japanese Research Collaboration in Medical ICT’ held at Hokkaido University, Japan (Aran Lunzer and Yuzuru Tanaka- Hokkaido University)
In September 2009 ACGT partner Hokkaido University hosted a two-day workshop that brought ACGT’s technical, medical and legal representatives together with planners from the Japan Science and Technology Agency (JST), and the leaders of academic and industrial research teams. As well as disseminating the EU's clinical-trial infrastructure strategy, as embodied in ACGT, the workshop provided a forum for discussing increased cooperation between Japan and the EU on future medical ICT (Information and Communication Technologies) projects. It is still rare for a European Commission-funded project to include a Japanese research partner. Although participation from outside Europe has been allowed since the Fourth Framework Programme (FP4), under FP6 there was a Japanese partner in only ten projects under the Information Society Technologies theme, of which just seven (including ACGT) are Integrated Projects. One barrier to participation is that partners in Japan cannot receive any EC funding; Hokkaido University has funded its work in ACGT using separately obtained competitive research grants from the Japanese government. Hokkaido University, based in Sapporo, is one of Japan’s seven former “imperial universities”, which also include Kyoto University and the University of Tokyo. When the proposal for ACGT was put together in 2005, Professor Yuzuru Tanaka’s Meme Media Laboratory had been collaborating with the technical leaders, FORTH, for over ten years, including numerous researcher exchanges. In 2004, Tanaka had helped set up a new graduate school whose mix of computer science and bioinformatics expertise gave it a clear fit with ACGT’s mission. [...] ACGT people ‘Workshop on European-Japanese Research Collaboration in Medical ICT’ held at Hokkaido University, Japan (Aran Lunzer and Yuzuru Tanaka- Hokkaido University)In September 2009 ACGT partner Hokkaido University hosted a two-day workshop that brought ACGT’s technical, medical and legal representatives together with planners from the Japan Science and Technology Agency (JST), and the leaders of academic and industrial research teams. As well as disseminating the EU's clinical-trial infrastructure strategy, as embodied in ACGT, the workshop provided a forum for discussing increased cooperation between Japan and the EU on future medical ICT (Information and Communication Technologies) projects. It is still rare for a European Commission-funded project to include a Japanese research partner. Although participation from outside Europe has been allowed since the Fourth Framework Programme (FP4), under FP6 there was a Japanese partner in only ten projects under the Information Society Technologies theme, of which just seven (including ACGT) are Integrated Projects. One barrier to participation is that partners in Japan cannot receive any EC funding; Hokkaido University has funded its work in ACGT using separately obtained competitive research grants from the Japanese government. Hokkaido University, based in Sapporo, is one of Japan’s seven former “imperial universities”, which also include Kyoto University and the University of Tokyo. When the proposal for ACGT was put together in 2005, Professor Yuzuru Tanaka’s Meme Media Laboratory had been collaborating with the technical leaders, FORTH, for over ten years, including numerous researcher exchanges. In 2004, Tanaka had helped set up a new graduate school whose mix of computer science and bioinformatics expertise gave it a clear fit with ACGT’s mission. [...] |