Enhancing Access to Cancer Clinical Trials
The Education Network to Advance Cancer Clinical Trials (ENACCT) is a US-based nonprofit organization, founded 2004 with support from the Lance Armstrong Foundation. ENACCT’s mission is to improve access to cancer clinical trials through education and collaboration with communities, health care providers, and researchers. ENACCT helps patients, communities, and health care providers better understand the importance of cancer clinical trials—while also helping researchers communicate more effectively about these trials. In this article, I am pleased to share ENACCT’s perspective on what it takes to enhance access to cancer clinical trials.
1. Work to Create Community Literacy About Clinical Research:
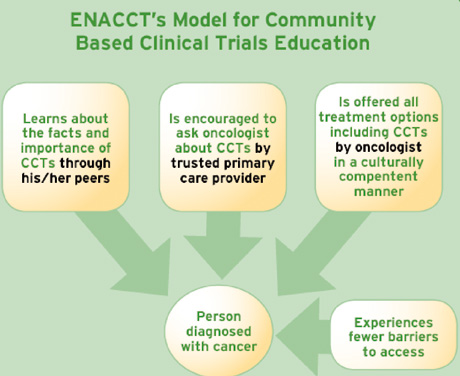
We need to consider that the public knows very little about clinical trials, and what it knows is extremely negative. At ENACCT, we believe that the optimal “teachable moment” may not be at the time of diagnosis, but may be at other times, such as churches, health fairs or community events, to help everyone understand that when a mother, sister or neighbor is diagnosed with cancer, she should ask her doctor, “Is there a clinical trial that’s right for me?” Considering the patient in the center of our educational programs as illustrated in the diagram below, ENACCT has developed a set of unique training programs to help
a) Community leaders reach their peers with simple messages focusing on the importance of clinical trials as a part of quality care.
b) Primary care providers talk with their patients prior to referral to oncology about the importance of clinical trials as a quality treatment option
c) Clinical trial staff to better conduct outreach, recruitment and retention for their trials.
While most of our programs are delivered live, we have a number of free on line courses that include Continuing Education Units for physicians, nurses and social workers. See www.enacct.org/ourprograms/ your-role-cancer-clinical- trials for more information.
Since founding the US-based ENACCT in 2004 with funding from the Lance
Armstrong Foundation, Margo Michaels has been the organization’s Executive
Director, whose mission is to improve access to cancer clinical trials through
education and collaboration with communities, health care providers, and
researchers.
2. Consider how clinical trial messages are portrayed in publications
Many publications discuss treatment as somehow “separate and apart from” clinical trials. However, for many people with cancer, clinical trials can be a viable treatment option. Why do we always save discussion on this issue to the moment of “last resort?” Take a look at how your own organization discusses clinical trials. We need to make sure that language we use helps give confidence to all patients to ask their physician, at all stages of treatment, “is there a clinical trial for me?”
3. Consider how patient advocates and community representatives can become more involved in clinical trials design and implementation
Less than three percent of all adult cancer patients participate in clinical trials. According to recent research by Dr. David Dilts from Vanderbilt University, between 50 and 60 percent of all the trials entered in US based cancer centers accrue fewer than five patients, and approximately one quarter accrues no patients! To address these concerns, a number of reports have called for greater inclusion of public representatives in clinical research design and implementation.
In 2007, we spearheaded a new effort called Communities as Partners in Cancer Clinical Trials: Changing Research, Practice and Policy, along with Community-Campus Partnerships for Health (CCPH), with core funding from the US government. Our final report highlights 58 recommendations for community engagement in the cancer clinical research process – from trial design to implementation to dissemination of results -- in order to address low accrual rates in cancer clinical trials-- and ultimately improve research outcomes. It is available at www.enacct.org. Our report is the first to detail why and how the cancer clinical trial process can involve communities affected by cancer– from trial design to implementation to dissemination of results - with a focus on community engagement strategies. We encourage you to “lift” the ideas that work for you and your country.
Margo Michaels,
Executive Director ENACCT
 In ENACCT communities, we hope to address patients’ lack of awareness about clinical research through reaching the groups that he or she is likely to encounter throughout the diagnostic process. The newly diagnosed patient will a) first hear about clinical trials through his/her peers, whether in the neighborhood, at the beauty shop, at church, or through an advocacy group; b) will then learn about the option of receiving treatment through a clinical trial by a trusted primary health care provider; c) will be better able to initiate inquiries about clinical trials and d) will be offered the opportunity to join a clinical trial by the oncologist, if he/she is eligible.
In ENACCT communities, we hope to address patients’ lack of awareness about clinical research through reaching the groups that he or she is likely to encounter throughout the diagnostic process. The newly diagnosed patient will a) first hear about clinical trials through his/her peers, whether in the neighborhood, at the beauty shop, at church, or through an advocacy group; b) will then learn about the option of receiving treatment through a clinical trial by a trusted primary health care provider; c) will be better able to initiate inquiries about clinical trials and d) will be offered the opportunity to join a clinical trial by the oncologist, if he/she is eligible.

