ObTiMA: Ontology Based Trial Management for ACGT
Data Management in post-genomic clinical trials is the process of collecting and validating clinical and genomic data with the goal to answer research questions and to preserve them for future scientific investigations. Comprehensive metadata describing the semantics of the data is needed to allow cross-trial analysis. Current clinical trial management systems lack sufficient metadata and are not semantically interoperable. ObTiMA is an application that allows trial chairmen to design their trial according to their needs and to integrate a clinical trial ontology into the design process
ObTiMA will support the design phase of a clinical trial. It will allow clinical trial chairmen to capture data definition and further design specifications for a clinical trial in a standardized way based on a formal ontology. From these definitions the database for the trial can then be set up automatically. The Trial Builder will be a component based extendable application. Important components are the CRF-Builder, the Trial Outline Builder, the data management system, a roles and rights management system and security features for anonymization or pseudonymization of personal data.
A variety of data has to be captured on CRFs during the conduction of the trial (e.g. patient's history, medical findings, diagnostic data or genomics data) and can be queried afterwards. The challenge is to define comprehensive metadata for the CRFs as well as for the clinical trial database in a way that equivalent data in different trials are described in the same manner and furthermore that this task can be conducted by a trial chairman. We address this by describing the content of the CRFs completely in terms of an ontology.
1. Creating the CRFs
A clinician aiming to design a trial does not want to be bothered with databases and an ontology application. Therefore the trial chairman will built CRFs by designing them from the domain ontology. The trial chairman can define the questions on the CRFs, their order and constraints regarding answer possibilities. To describe answers semantically, a description from the ontology has to be chosen. Although an ontology is "human understandable" by providing natural language definitions of entities and relationships it is by definition not based on practical or clinical perceptions of reality. The ACGT-MO is moreover based on description logics and therefore hard to understand by clinical users. Therefore a tool is implemented that provides a clinical view (CV) on the ontology. The CV is integrated in the CRF Builder component in a way that it will guide the trial chairman to select appropriate paths to describe the questions on his CRFs from the ontology.
2. Output of the Trial Builder
The output of the Trial Builder is a platform independent description of the metadata and the CRFs for the clinical trial. This description will be stored in an XML format that is based on the Operational Data Model (ODM) from the Clinical Data Interchange Standards Consortium (CDISC). ODM is a vendor neutral, platform independent format for interchange and archive of clinical trials data. The metadata of a clinical trial including a full description of the CRFs of the trial can be stored in the ODM format.
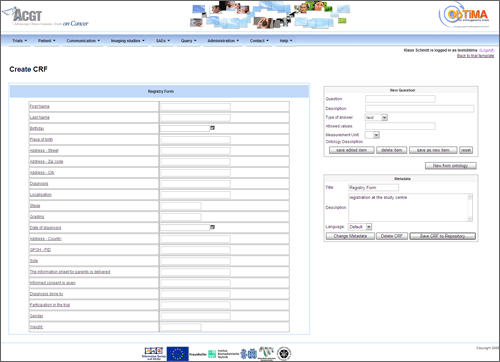
Figure 1. Screenshot of ObTiMA
3. CRF Repository
Since in many trials similar or equal data is collected, a crucial part of the system is a CRF repository. This repository will allow to store the developed CRFs or parts of them to reuse them in later trials. This is important to share CRFs between similar clinical trials and to develop a set of standardized CRFs. Semantic search based on the ontology will be possible in the CRF repository.
Trial Outline Builder
The Trial Outline builder of ObTiMA provides the whole functionality of the tool via a graphical interface. This allows trial chairmen to built, run and analyze trials intuitively. Such a component is completely new to trial management systems.
Roles and Rights Management and Data Security
ObTiMA contains a roles and rights management system and features to guarantee data security and possibilities for anonymization or pseudonymization of personal data. An audit trail and other features are in compliance with GCP criteria.
Conclusion
The described approach of enabling trial leaders to set up clinical data management systems with comprehensive metadata by integrating an ontology in the design process has several advantages. It will enable trial chairmen to create reusable CRFs that allow the collection of standardized data based on an underlying ontology. After anonymization such data can be directly shared and integrated in other systems using the same reference ontology for easy querying and analyzing. The described tools are under development. A first prototype will be available by the end of 2009.
This article is based on the following publication:
Weiler G, Brochhausen M, Graf N, Hoppe A, Schera F, Kiefer S: Ontology Based Data Management Systems for post-genomic clinical Trials within an European Grid Infrastructure for Cancer Research. Proceedings of the 29th Annual International Conference of the IEEE EMBS, Cit? Internationale, Lyon, France, August 23-26, 2007, SuA11.4; Conf Proc IEEE Eng Med Biol Soc. 2007;1:6434-6437
Norbert Graf,
Uniklinikum Saarland.

